How to Join the Study?
Speak with your doctor or specialist to see if you qualify and request a referral to a participating trial site.
About the CONVERT II Study
About the
CONVERT II Study
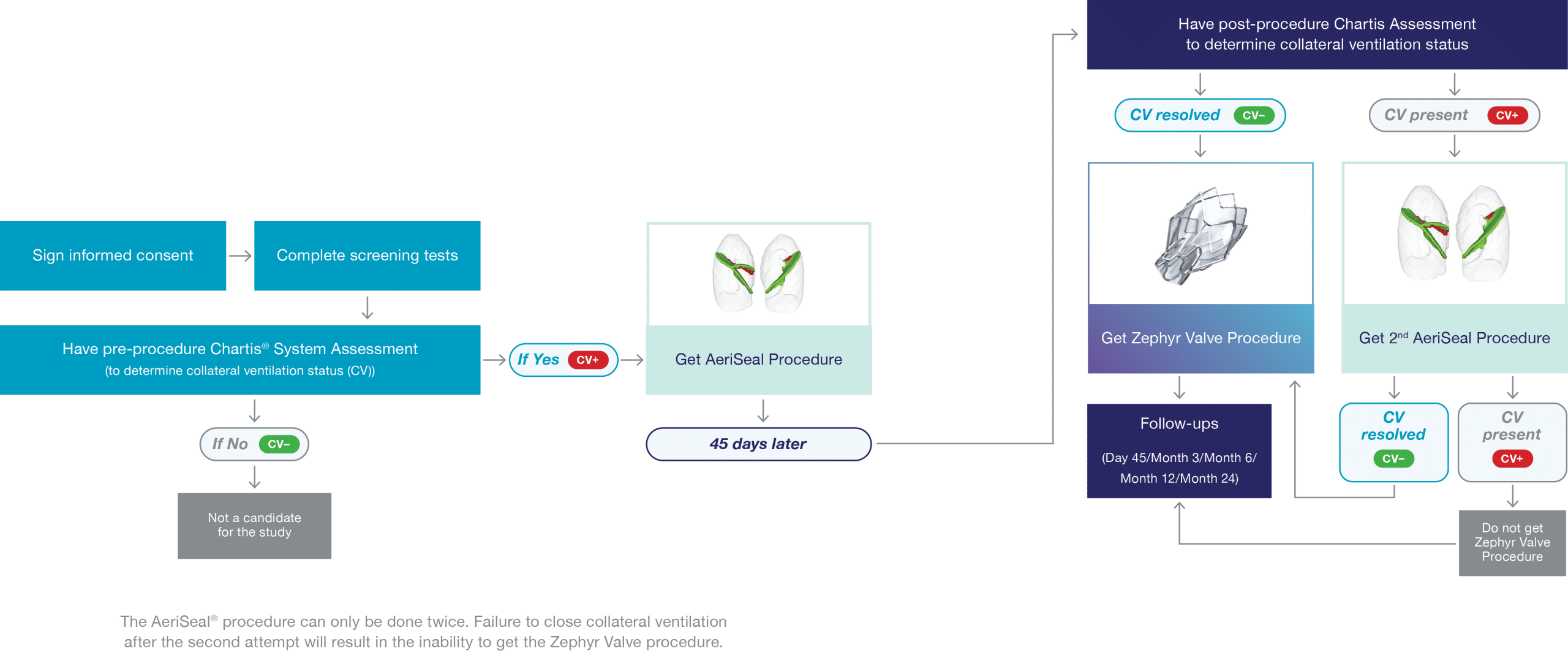
The CONVERT II Trial (NCT06035120) is an international study evaluating the AeriSeal® System’s ability to limit collateral ventilation (CV) in patients with severe COPD/emphysema. Collateral ventilation occurs due to naturally occurring openings in the lung fissures, preventing some patients from benefiting from bronchoscopic lung volume reduction (BLVR) with endobronchial valves (EBVs), a minimally invasive treatment.
The AeriSeal System is designed to block these openings in the targeted lobe, effectively stopping collateral ventilation. Successful treatment with the AeriSeal System is followed by placement of endobronchial valves, a guideline-based treatment for patients with severe emphysema/COPD.
Bronchoscopic lung volume reduction (BLVR) with EBVs is a minimally invasive procedure to reduce hyperinflation and improve lung function and quality of life. During the procedure, a small, flexible tube, called a bronchoscope is inserted in the mouth or nose into the most diseased part of the lung. A delivery catheter is used to implant the valves to block the flow of air into this part of the lung. These one-way valves allow trapped air to escape while preventing new air from entering thereby reducing the size of the diseased part of the lung. This allows the remaining lung to function better, making breathing easier and reducing shortness of breath. The procedure requires no cutting or incisions and takes about 1 hour.1
Risks
All medical and surgical procedures can have side effects. Refer to the Informed Consent for a detailed list of potential side effects. Participants may have none, some, or all of the effects listed, and they may be mild, moderate, or severe. If you have any of these side effects, or are worried about them, talk with the study doctor. The study doctor will also be looking out for side effects.
Benefits
Potential benefits from participation in the study may include improved lung function and other positive changes that may be associated with improved quality of life.
While no benefit is guaranteed, it is possible that your severe COPD/emphysema may get better. It is also possible that your condition may remain unchanged or get worse; we hope the information learned from this research study will benefit other individuals with severe COPD/ emphysema in the future.
What will happen during the study?
If you consent to enrolling in the CONVERT II study, you will have a series of tests and assessments to ensure eligibility.
Participants will be evaluated at 14 visits (9 in clinic and 5 via phone) over 2½ years. In addition, pulmonary rehab must be completed within 12 months of enrolment and following treatment with endobronchial valves.
About the
CONVERT II Study
The CONVERT II Trial (NCT06035120) is an international study evaluating the AeriSeal® System’s ability to limit collateral ventilation (CV) in patients with severe COPD/emphysema. Collateral ventilation occurs due to naturally occurring openings in the lung fissures, preventing some patients from benefiting from bronchoscopic lung volume reduction (BLVR) with endobronchial valves (EBVs), a minimally invasive treatment.
The AeriSeal System is designed to block these openings in the targeted lobe, effectively stopping collateral ventilation. Successful treatment with the AeriSeal System is followed by placement of endobronchial valves, a guideline-based treatment for patients with severe emphysema/COPD.
Bronchoscopic lung volume reduction (BLVR) with EBVs is a minimally invasive procedure to reduce hyperinflation and improve lung function and quality of life. During the procedure, a small, flexible tube, called a bronchoscope is inserted in the mouth or nose into the most diseased part of the lung. A delivery catheter is used to implant the valves to block the flow of air into this part of the lung. These one-way valves allow trapped air to escape while preventing new air from entering thereby reducing the size of the diseased part of the lung. This allows the remaining lung to function better, making breathing easier and reducing shortness of breath. The procedure requires no cutting or incisions and takes about 1 hour.1
Risks
All medical and surgical procedures can have side effects. Refer to the Informed Consent for a detailed list of potential side effects. Participants may have none, some, or all of the effects listed, and they may be mild, moderate, or severe. If you have any of these side effects, or are worried about them, talk with the study doctor. The study doctor will also be looking out for side effects.
Benefits
Potential benefits from participation in the study may include improved lung function and other positive changes that may be associated with improved quality of life.
While no benefit is guaranteed, it is possible that your severe COPD/emphysema may get better. It is also possible that your condition may remain unchanged or get worse; we hope the information learned from this research study will benefit other individuals with severe COPD/ emphysema in the future.
What will happen during the study?
If you consent to enrolling in the CONVERT II study, you will have a series of tests and assessments to ensure eligibility.
Participants will be evaluated at 14 visits (9 in clinic and 5 via phone) over 2½ years. In addition, pulmonary rehab must be completed within 12 months of enrolment and following treatment with endobronchial valves.

Who Can Participate?
You may be eligible if:
- You are between 40 and 80 years old
- You have been diagnosed with severe emphysema/COPD
- You have stopped smoking for at least 8 weeks before enrolling
Who Can Not Participate?
You may not be eligible if:
- You have had prior lung surgery or a lung transplant
- You have been hospitalized three or more times in the past year due to emphysema/COPD
- Your primary diagnosis is asthma or chronic bronchitis
- You require invasive ventilatory support
If you’re unsure about your eligibility, speak to your doctor.
About Emphysema & COPD
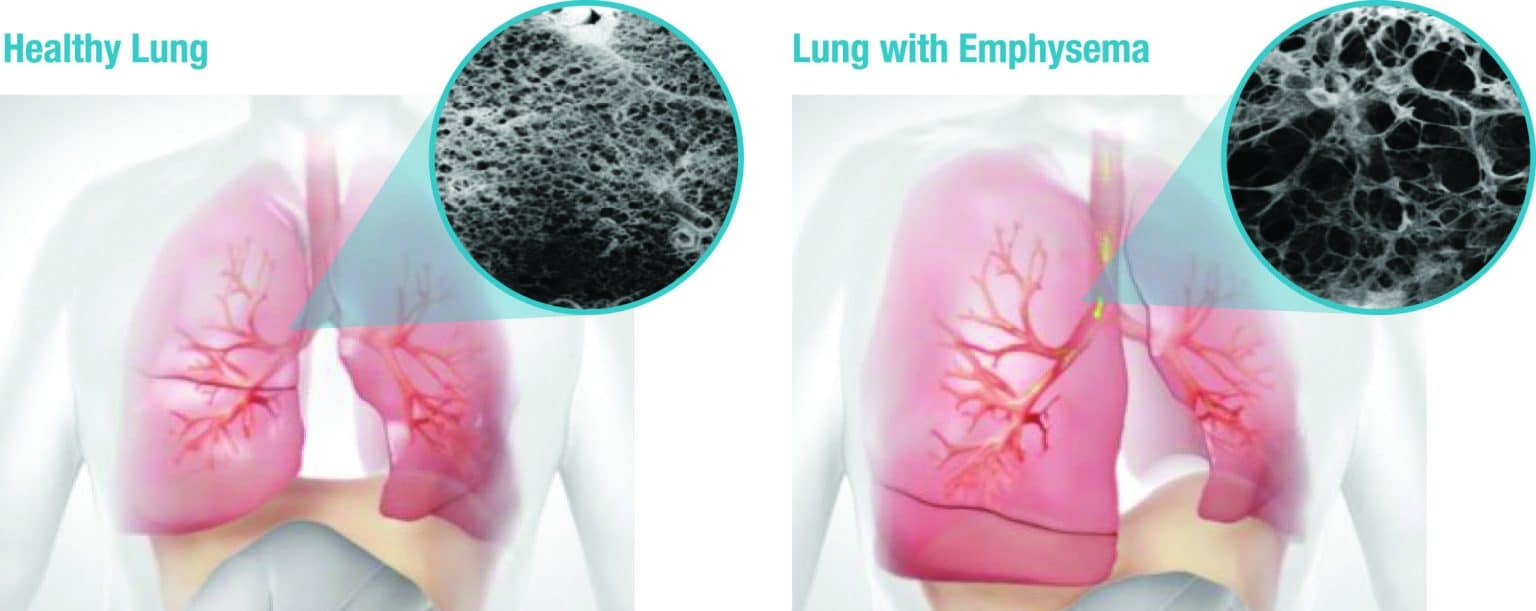
COPD stands for chronic obstructive pulmonary disease — a common condition affecting the airways. It is caused by pollutants that get into the lungs, initially blocking (obstructing) the airways as well as causing chronic inflammation. The main cause is smoking. The urge to cough caused by persistent bronchial irritation is commonly referred to as smoker’s cough. Damage to the bronchi that has become irreversible is referred to by lung specialists as a permanent obstruction to the airways, i.e., COPD.
Symptoms of Emphysema
- Becoming short of breath easily when you do everyday things such as going for a walk or doing housework
- Exacerbation (obvious worsening of the condition)
- Coughing up sputum or phlegm
- Persistent cough
- Wheezing
- Frequent airway infections, such as bronchitis or pneumonia
- Difficulty performing daily activities and enjoy many aspects of life without stopping for breath, taking a break, or asking for help.
Disease Progression
Stage 1
Mild
You may have no symptoms, or you may get winded with moderate exercise or when walking upstairs. Your pulmonary function test indicates that your airflow is about 80% of normal.
Stage 2
Moderate
You may need to stop and catch your breath when walking on level ground. You may complain of coughing or wheezing, and breathlessness. Your pulmonary function test indicates that your airflow is about 50% to 79% of normal.
Stage 3
Severe
Your shortness of breath is limiting your life and daily activities, with worsening symptoms. Your pulmonary function test indicates that your airflow is about 30% to 50% of normal. You may need additional tests at this stage to see how your lungs are functioning.
Stage 4
Very Severe
Known as end-stage COPD, your oxygen levels are low due to lack of airflow. You will have difficulty catching your breath, even at rest. You may also have severe flare-ups causing frequent hospitalizations. These flare-ups may be life- threatening. Your pulmonary function test indicates that your airflow is less than 30% of normal.
Treatment Options for Severe COPD/Emphysema
Severe COPD/emphysema is a progressive disease and gets worse over time. There is currently no cure for this condition, but treatment options exist. Less invasive options include medication, often delivered via an inhaler. This is usually the first thing your doctor will suggest, along with smoking cessation. But as the condition progresses, some medications that have worked in the past may stop being effective. For some people, medication and oxygen therapy do not provide enough relief.
Together with your primary healthcare provider, which could be your general practitioner or respiratory physician, you can create a treatment plan that works for you.
Non-Invasive
Non-surgical options include COPD medicines, such as inhalers and oral steroids, stopping smoking programmes, pulmonary rehabilitation and oxygen therapy.
COPD medicines
These may include inhalers, oral steroids, antibiotics, or other prescription drugs.
Stop smoking programmes
Smoking cessation programmes help you quit smoking to prevent further damage to your lungs.
Rehabilitation
Your doctor can recommend a rehabilitation course to help you exercise your lungs and learn to breathe more efficiently.
Oxygen therapy
Emphysema reduces the amount of oxygen that reaches your bloodstream. Your doctor can prescribe you oxygen therapy if medication does not help you enough.
If you’ve stopped smoking, or are willing to stop, and medical management is no longer working, you may be suitable for a minimally invasive option called endobronchial valve treatment.
Minimally Invasive Bronchoscopic
Lung volume reduction procedure with endobronchial valves
Bronchoscopic or minimally invasive lung volume reduction procedure with endobronchial valves. Endobronchial valves are a breakthrough technology that is clinically proven to help select patients breathe easier, be more active, and enjoy a better quality of life1.
The endobronchial valves are not another medication or surgery. It is a minimally invasive procedure that uses a bronchoscope to place small one-way valves in a targeted, diseased lobe of your lung where trapped air is causing shortness of breath. The endobronchial valves allow trapped air from your treated lung to escape while preventing air from entering that lung lobe and so hyperinflation in the lung is reduced. Reducing this hyperinflation allows the healthier parts of your lung to expand and function better.
Surgical
Lung volume reduction with surgery and bullectomy
The operation is usually performed during a procedure called VATS (video assisted thoracoscopic surgery). The surgeon makes a small cut in one side of your chest. A special instrument is used to both cut a diseased portion of the lung and staple the lung at the same time. The staple line seals the cut surface and prevents or reduces any air leaks.
Lung transplant
When the lungs are too damaged to benefit from surgery, certain patients may meet the criteria for lung transplantation surgery.
Participating Trial Sites & Contact Information
How to Join the Study?
Speak with your doctor or specialist to see if you qualify and request a referral to a participating trial site.
New South Wales
Professor Alvin Ing
Macquarie University Clinic
Suite 306, Level 3, 2 Technology
Macquarie University, NSW 2109
Contact: [email protected]
Queensland
Dr Farzad Bashirzadeh
Wesley Research Institute
Level 8, East Wing, The Wesley Hospital
451 Coronation Drive, Auchenflower, QLD 4066
Contact: [email protected]
South Australia
Professor Phan Nguyen
Royal Adelaide Hospital
Department of Thoracic Medicine
Port Road, Adelaide, SA 5000
Contact: [email protected]
References: 1. Criner, G et al. Am J Resp Crit Care Med. 2018; 198(9):1151‒1164
The CONVERT II Study has been granted ethics approval by the Macquarie University Clinic Human Research Ethics Committee (HREC), the Wesley Research Institute Human Research Ethics Committee, and the Royal Adelaide Hospital Human Research Ethics Committee.
The study will be conducted in accordance with the Declaration of Helsinki, the guidance of the International Council on Harmonization (ICH) Guidelines for Good Clinical Practice (E6 R2), the MDR Regulations (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, Title 21 of the FDA Code of Federal Regulations, Good Clinical Practice (Parts 812, 11, 50, 54, 56), Clinical investigation of medical devices for human subjects – Good clinical practice (ISO 14155:2020), and other applicable local and federal regulations, including the archiving of essential documents.
Sponsor: Pulmonx Australia – Suite 5, Level 6 65 York Street Sydney, NSW 2000 Australia. P: 1300 785 666 E: [email protected] Visit our website: pulmonx.com.au
© 2025 Pulmonx Corporation or its affiliates. All rights reserved. All trademarks herein are the property of Pulmonx Corporation and its affiliates CL159EN_A 4/04/2025.
